What Relationship Does Avogadros Law Describe
A modern statement is. These laws help to explain the relationship that gases have between the number of molecules and the volume of the container they fill.
Avogadro S Law Study Guide Inspirit
The law states that equal volume of all gases at the same temperature and pressure contains the same number of.

. Avogadros law is commonly known as Avogadros principle or Avogadros hypothesis. This empirical relation can be derived from the kinetic theory of gases under the assumption of a perfect ideal gas. Equal volumes of gases at the same temperature and pressure have an equal number of molecules.
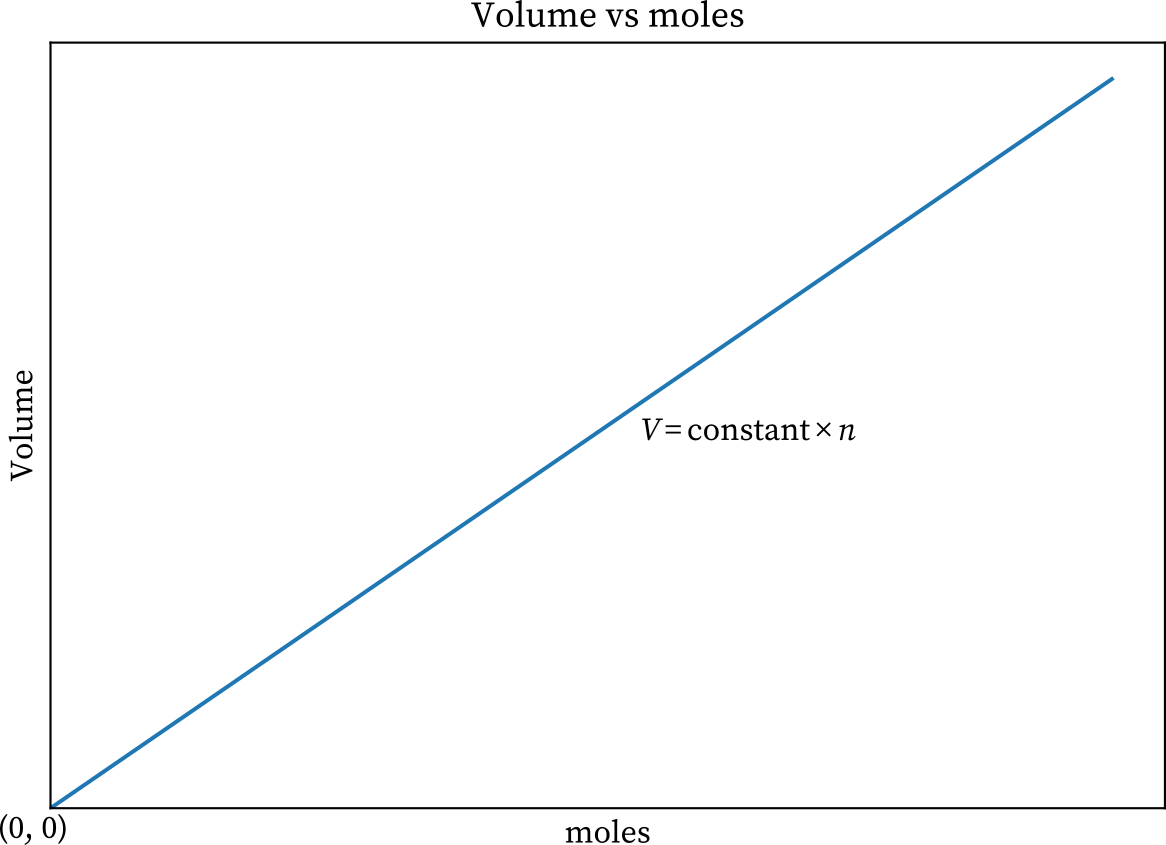
The link between the amount of gas n and the volume V is investigated via Avogadros law v. Apex The relationship between volume and moles Explanation Avogadros law describes the above relationship when pressure and temperature are held constant. Avogadros law states that equal volumes of all gases at the same temperature and pressure have the same number of molecules For a given mass of an ideal gas the volume and amount moles of the gas are directly proportional if the temperature and pressure are constant.
The Avogadro law is. Avogadros law states that equal volumes of all gases at the same temperature and pressure have the same number of molecules For a given mass of an ideal gas the volume and amount moles of the gas are directly proportional if the temperature and pressure are constant. The law is approximately valid for real gases at sufficiently low pressures and high temperatures.
Avogadros law now known as Avogadros hypothesis was first published in 1811 and is one of the main theories that helped to build the foundation for the ideal gas laws. Amedo Avogadro found the relationship between the volume of a gas and the number of molecules contained in the volume. The law is significant because it allows us to save time and money over time.
According to Avogadros Hypothesis other conditions remaining identical equal volumes of all gases will contain same no. Equal volumes of gases at the same temperature and pressure have an equal number of molecules. No-Name Law The relationship between pressure and temperature.
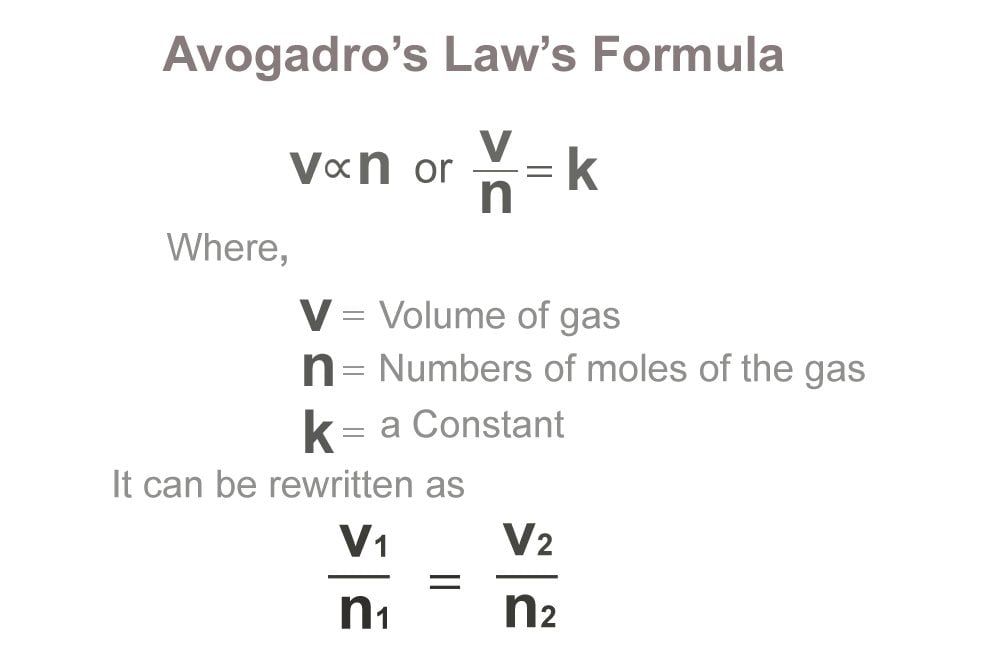
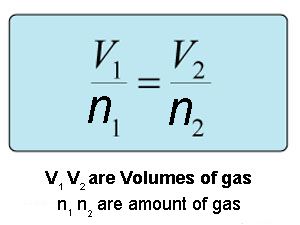
What relationship does Avogadros law describe. A mathematical expression that summarizes Avogadros law is Explanation. What must remain constant for Avogadros Law pressure and temp equation V1N1 V2N2 Which of the following is a variable in Avogadros Law but not in.
We can express this mathematically as. Avogadros law states that at constant temperature and pressure the volume of a gas is directly proportional to the number of moles present. The law is important because helps us save time and money in the long-run24 мая 2015 г.
Avogadros law investigates the relationship between the amount of gas n and volume v. Its a direct relationship meaning the volume of a gas is directly propotional to the number of moles the gas sample present. What relationship does Avogadros law describe.
Avogadros law was originally. This law is directly related to the ideal gas equation as both link temperature. Avogadros Law the volume of a gas and the number of moles of a gas are directly related.
Amedo Avogadro found the relationship between the volume of a gas and the number of molecules contained in the volume. Hence the volume of a gas at constant pressure and temperature is directly proportional to the number of moles. The Avogadro law is.
What relationship does Avogadros law describe. Avogadros law states that the number of atomsparticles present in the gas is directly proportional to the volume consumed by the gas at constant temperature and pressure. Its a direct relationship which means the volume of a gas is proportional to the number of moles contained in the gas sample.
It is expressed as. 941 V n a t c o n s t a n t P a n d T 942 V c o n s t a n t n o r V n c o n s t a n t. Avogadros law The volume V of an ideal gas varies directly with the number of moles of the gas n when the pressure P and the number of temperature T are constant.
What is Avogadros law class 11. Avogadros Law states that the volume of gas substances is directly proportional to the their amount in number of moles at constant temperature and pressure. Avogadros law a statement that under the same conditions of temperature and pressure equal volumes of different gases contain an equal number of molecules.
Comments
Post a Comment